MENU
US | USD
US | USD
-
- Benchtop Centrifuges
- Floor-Standing Centrifuges
- Refrigerated Centrifuges
- Microcentrifuges
- Multipurpose Centrifuges
- High-Speed Centrifuges
- Ultracentrifuges
- Concentrator
- High-Speed and Ultracentrifugation Consumables
- Accessories
- Tubes
- Plates
- Device Management Software
- Sample and Information Management
No results found
Search Suggestions
How Does Mycoplasma Contamination Affect Cell Culture?
Lab Academy
- Cell Biology
- Cell Culture
- Stem Cells
- Safety & Biosafety
- Reproducibility
- Contamination
- CO2 Incubators
- Essay
This article was published first in " Inside Cell Culture " , the monthly newsletter for cell culture professionals. Find more interesting articles about CO2 incubators on our page " FAQs and material on CO2 incubators " .
Read more
Read less
Mycoplasma: the silent but deadly threat to cell culture
Microbial contamination is arguably the biggest threat to mammalian cell cultures, risking the loss of valuable time, money, and resources. Bacteria belonging to the Mycoplasma genus are one of the most difficult microbial contaminants to control, estimated to affect over 10% of all cell cultures [1].
Over 100 Mycoplasma species have been characterized so far, although most Mycoplasma contamination in cell cultures is caused by a select few species, including M. arginini, M. pirum, M. orale, M. hominis, M. fermentans, M. salivarium and Acholeplasma laidlawii, each of which can infect a wide range of tissue types and mammalian species [2, 3, 4].
In the lab, there are several different ways that these Mycoplasma species can contaminate cell cultures, including via the use of contaminated animal sera, cell media, or reagents. Humans are another major source of contamination, with improper septic technique leading to Mycoplasma that may be present on the skin, clothing, or from airborne particles entering the cell cultures [5].
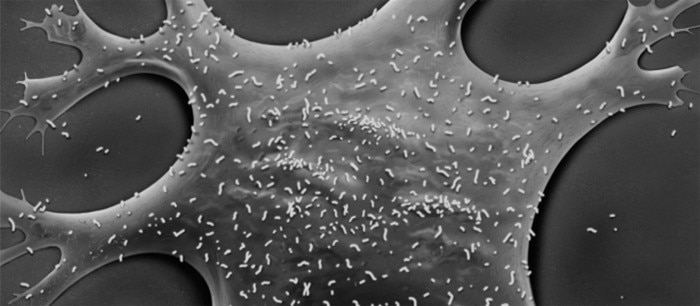
Measuring in at less than 300 nm, Mycoplasma is the smallest self-replicating organism discovered to date [6]. Their small size and lack of cell wall makes them hard to identify under a conventional light microscope. Instead, Mycoplasma can only be imaged using more sophisticated microscopy techniques, such as scanning electron microscopy (SEM) [7].
Read more
Read less

Figure 1: Scanning electron microscopy (SEM) image of Mycoplasma infected fibroblasts.
Adding fuel to the fire, Mycoplasma contamination often causes no visible signs of infection. Unlike other microbial contaminants, these bacteria don’t cause turbidity in the growth medium, meaning they often go undetected in cell culture. To help control this problem, several methods for Mycoplasma prevention and detection have been developed, and you can learn more about them here.
Read more
Read less
Tiny cell hijackers
It’s important to know the effects that Mycoplasma contamination can have on your cell cultures, to help you spot the signs of infection, and understand how your experimental results will be affected.
With an extremely simple genome, this bacterial pathogen is unable to synthesize nutrients itself, relying completely on its hosts to meet its metabolic needs. Consequently, Mycoplasma consume nutrients within the cell culture media, outcompeting host cells for essential nutrients, such as amino acids, fatty acids, sugars, nucleic acid precursors, and choline, which can result in a wide range of cellular defects.
Mycoplasma also employ complex mechanisms to aid the infection of host cells. For instance, Mycoplasma such as Acholeplasma laidlawii adhere to the surface of their host cells and secrete extracellular vesicles that are transported across the cell membrane and into the host (Figure 2). These vesicles have been shown to modulate gene expression and cause several other cellular defects [8, 9].
In this article, we’ll take a closer look at the common changes to cells that occur during Mycoplasma infection because of the secretion of modulatory compounds and nutrient starvation, and what this could mean for your cultures.
1. Altered gene expression
Mycoplasma induce widespread genetic dysregulation in their hosts. This occurs through the secretion of DNA methyltransferases, which selectively methylate DNA sites to repress the expression of a wide range of genes across the host’s genome [10].
In human cells, DNA methyltransferases that are secreted by Mycoplasma have been shown to activate cancer-associated genes, subsequently altering cell signaling pathways that are associated with cell proliferation, development, and survival [10]. Consequently, the secretion of Mycoplasma DNA methyltransferases induces widespread genetic and cellular effects within infected cell cultures.
Gene dysregulation can also occur through several other mechanisms including DNA degradation and chromosomal aberrations, which are discussed in more detail below. As a result of these mechanisms, Mycoplasma alter the expression of hundreds of genes across several key signaling pathways and stress responses, such as cytokinin signaling, apoptosis, DNA damage, and cell cycle signaling [11, 12].
Mycoplasma infection can therefore have several negative effects on your cell cultures, including interference with cell screening assays, disruption of the production of proteins such as enzymes and antibodies, and compromised validity of your experimental results.
2. DNA degradation
Mycoplasma are unable to synthesize nucleic acid precursors, such as purine and pyrimidine bases, that are essential to DNA and RNA synthesis [13]. Instead, these pathogens degrade the genetic material of their hosts to acquire nucleic acid precursors [14].
This is achieved through the secretion of nucleases that induce internucleosomal DNA degradation in host cells, leading to harmful cellular defects including altered gene regulation and apoptosis [15]. This reduces the longevity of your cultures, risking the loss of precious cell lines.
3. Chromosome aberrations
Chromosomal aberrations are caused by both fermenting and non-fermenting Mycoplasma species, although those that are induced by non-fermenting Mycoplasma species are more characterized. These non-fermenting Mycoplasma use the host’s stores of arginine as an essential energy source, secreting arginine deiminase to hydrolyze arginine and form ATP. Consequently, this amino acid is often depleted in cell cultures that are contaminated with Mycoplasma.
Arginine depletion inhibits histone production and causes chromosomal defects in cell cultures, including permanent chromosome loss. This can reduce genetic stability, posing a threat to the integrity and safety of cell cultures and their expressed products.
Several additional chromosome alterations during Mycoplasma infection occur, including chromatid breaks, endoreduplications, translocations, acentric fragmentation, and the formation of dicentric chromosomes [16]. These aberrations commonly disrupt exons and result in the creation of fusion genes, leading to severe cellular defects that can further harm the quality and productivity of your cell cultures [17].
4. Alterations to cell physiology
In addition to chromosome aberrations, utilization of the host’s arginine causes several other cellular changes during Mycoplasma infection. For instance, the secretion of arginine deiminase can inhibit host cell proliferation by arresting the cell cycle in the G1 and G2 phases, with high concentrations of this enzyme also resulting in apoptosis [18].
Arginine depletion within infected cell cultures can also cause additional cellular defects, including reduced cell growth, viability and DNA transfection efficiency, increased cell granulation, and detachment from the cell culture vessel surface [5, 19].
The depletion of other essential host nutrients can also cause harmful effects to cell health. For example, the reduction of purine and pyrimidine in cell cultures can inhibit cell proliferation [20], and reduced choline levels can lead to apoptosis [21].
The widespread physiological changes that result from the depletion of arginine and other essential nutrients during Mycoplasma infection undoubtedly harm your experiments, impairing many aspects of cellular function to reduce cell viability and cause unreproducible and inaccurate results.
Read more
Read less
Tackle Mycoplasmas and protect your cell culture experiments
The evidence is clear − Mycoplasma infection causes severe defects to cultured cells, including altered gene expression, DNA degradation, and chromosome aberration, in addition to defects in cell physiology, including cell growth, metabolism, and behavior.
Read more
Read less
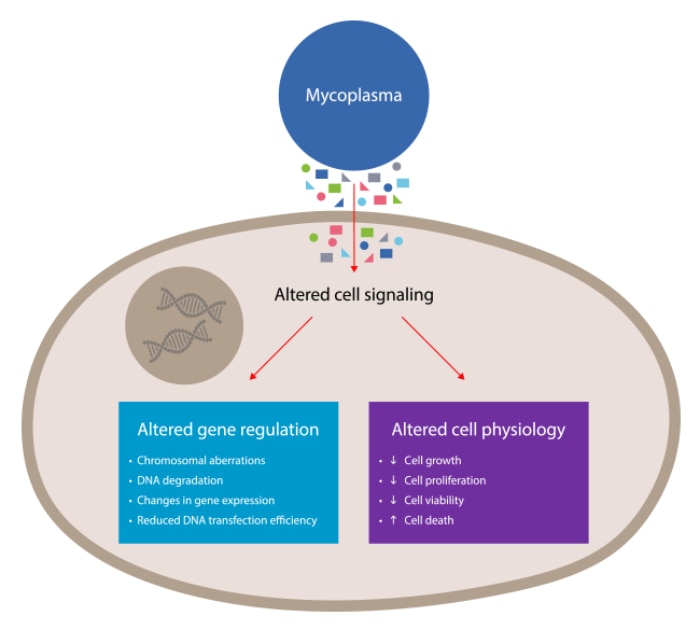
Figure 2. An overview of the severe cellular defects that Mycoplasma infection can cause on infected host cells. These result from the secretion of extracellular vesicles, and from the nutrient depletion that is caused by Mycoplasma outcompeting their host cells for essential nutrients, such as amino acids, fatty acids, sugars, nucleic acid precursors, and choline.
These changes to contaminated cell cultures will undeniably affect the validity and reproducibility of experimental results. This means that regularly testing your cell cultures for Mycoplasma contamination is vital to ensuring that your time, effort, and precious resources don’t go to waste.
Read more
Read less
Methods for Mycoplasma detection
There are several different methods for Mycoplasma detection, each with varying suitability for your lab workflows.
To help you choose which is best, we’ve listed five of the most commonly used Mycoplasma detection methods in Table 1 below, outlining their sensitivity and specificity, in addition to the advantages and disadvantages that you should consider:
Read more
Read less
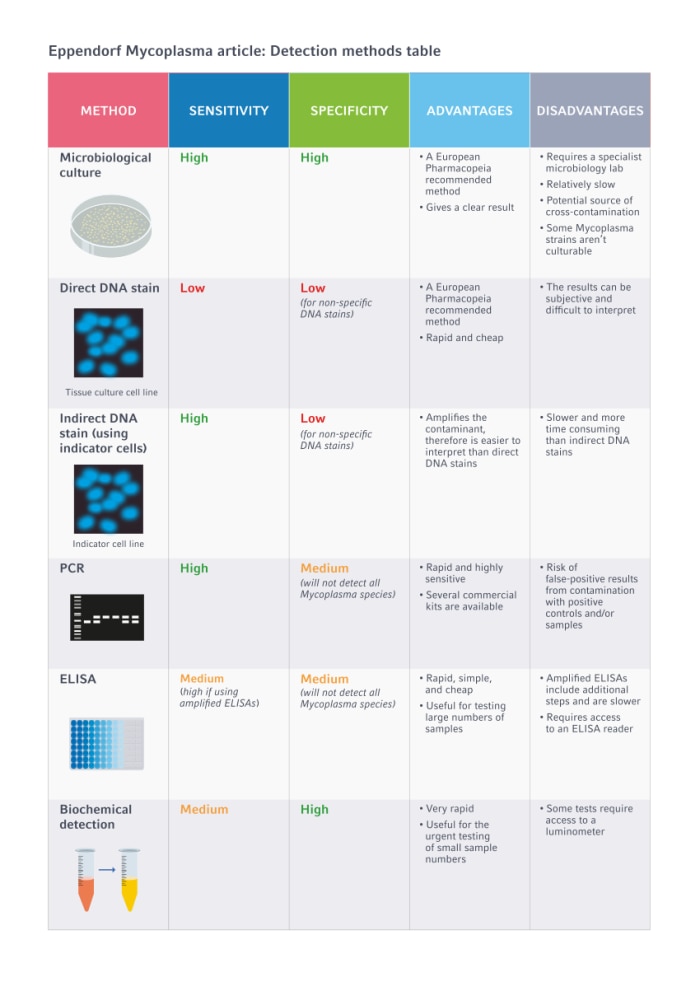
Table 1. Summary table of Mycoplasma detection methods, including their sensitivity, specificity, advantages, and disadvantages.
An integrated approach to Mycoplasma contamination
With Mycoplasma being invisible to the naked eye, and the wide range of genetic and cellular defects that this pathogen causes, routine testing isn’t the only step to helping ensure that your cell cultures remain Mycoplasma free.
An integrated approach is recommended, including carefully considered methods for prevention, detection, and elimination. For more information on controlling Mycoplasma contamination, visit our Mycoplasma contamination webpage .
Read more
Read less
References
[1] C. C. Uphoff and H. G. Drexler, "Comparative PCR analysis for detection of mycoplasma infections in continuous cell lines.," In vitro cellular & developmental biology. Animal, vol. 38, no. 2, pp. 79-85, February 2002.
[2] H. G. Drexler and C. C. Uphoff, "Mycoplasma contamination of cell cultures: Incidence, sources, effects, detection, elimination, prevention.," Cytotechnology, vol. 39, no. 2, pp. 75-90, July 2002.
[3] J. Timenetsky, L. M. Santos, M. Buzinhani and E. Mettifogo, "Detection of multiple mycoplasma infection in cell cultures by PCR.," Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas, vol. 39, no. 7, pp. 907-14, July 2006.
[4] J. Schmidt and V. Erfle, "Elimination of mycoplasmas from cell cultures and establishment of mycoplasma-free cell lines," Experimental cell research, vol. 152, p. 565–570, 1984.
[5] L. Nikfarjam and P. Farzaneh, "Prevention and detection of Mycoplasma contamination in cell culture.," Cell journal, vol. 13, no. 4, pp. 203-12, 2012.
[6] S. Razin, "Peculiar properties of mycoplasmas: the smallest self-replicating prokaryotes.," FEMS microbiology letters, vol. 100, no. 1-3, pp. 423-31, December 1992.
[7] P. Falagan-Lotsch, T. S. Lopes, N. Ferreira, N. Balthazar, A. M. Monteiro, R. Borojevic and J. M. Granjeiro, "Performance of PCR-based and Bioluminescent assays for mycoplasma detection," Journal of Microbiological Methods, vol. 118, pp. 31-36, 2015.
[8] V. M. Chernov, O. A. Chernova, J. T. Sanchez-Vega, A. I. Kolpakov and O. N. Ilinskaya, "Mycoplasma Contamination of Cell Cultures: Vesicular Traffic in Bacteria and Control over Infectious Agents.," Acta naturae, vol. 6, no. 3, pp. 41-51, July 2014.
[9] A. A. Mouzykantov, E. V. Rozhina, R. F. Fakhrullin, M. O. Gomzikova, M. A. Zolotykh, O. A. Chernova and V. M. Chernov, "Extracellular Vesicles from Mycoplasmas Can Penetrate Eukaryotic Cells In Vitro and Modulate the Cellular Proteome.," Acta naturae, vol. 13, no. 4, pp. 82-88, October 2021.
[10] A. V. Chernov, L. Reyes, Z. Xu, B. Gonzalez, G. Golovko, S. Peterson, M. Perucho, Y. Fofanov and A. Y. Strongin, "Mycoplasma CG- and GATC-specific DNA methyltransferases selectively and efficiently methylate the host genome and alter the epigenetic landscape in human cells.," Epigenetics, vol. 10, no. 4, pp. 303-18, 2015. [11] C. J. Miller, H. S. Kassem, S. D. Pepper, Y. Hey, T. H. Ward and G. P. Margison, "Mycoplasma infection significantly alters microarray gene expression profiles.," BioTechniques, vol. 35, no. 4, pp. 812-4, October 2003.
[12] C. Doyle, R. Nakamura, R. Bing, B. Rousseau and R. C. Branski, "Mycoplasma affects baseline gene expression and the response to glucocorticoids in vocal fold fibroblasts.," Journal of medical microbiology, vol. 70, no. 5, May 2021.
[13] J. D. Pollack, "The necessity of combining genomic and enzymatic data to infer metabolic function and pathways in the smallest bacteria: amino acid, purine and pyrimidine metabolism in Mollicutes.," Frontiers in bioscience : a journal and virtual library, vol. 7, pp. d1762-81, August 2002.
[14] L. Wang, J. Westberg, G. Bölske and S. Eriksson, "Novel deoxynucleoside-phosphorylating enzymes in mycoplasmas: evidence for efficient utilization of deoxynucleosides.," Molecular microbiology, vol. 42, no. 4, pp. 1065-73, November 2001.
[15] R. Paddenberg, A. Weber, S. Wulf and H. G. Mannherz, "Mycoplasma nucleases able to induce internucleosomal DNA degradation in cultured cells possess many characteristics of eukaryotic apoptotic nucleases.," Cell death and differentiation, vol. 5, no. 6, pp. 517-28, June 1998.
[16] R. B. Kundsin, M. Ampola, S. Streeter and P. Neurath, "Chromosomal aberrations induced by T strains mycoplasmas.," Journal of medical genetics, vol. 8, no. 2, pp. 181-7, June 1971.
[17] W. P. Kloosterman and R. Hochstenbach, "Deciphering the pathogenic consequences of chromosomal aberrations in human genetic disease.," Molecular cytogenetics, vol. 7, no. 1, p. 100, 2014.
[18] H. Gong, F. Zölzer, G. von Recklinghausen, J. Rössler, S. Breit, W. Havers, T. Fotsis and L. Schweigerer, "Arginine deiminase inhibits cell proliferation by arresting cell cycle and inducing apoptosis.," Biochemical and biophysical research communications, vol. 261, no. 1, pp. 10-4, July 1999.
[19] Z.-F. Yin, Y.-N. Zhang, S.-F. Liang, S.-S. Zhao, J. Du and B.-B. Cheng, "Mycoplasma contaminationmediated attenuation of plasmid DNA transfection efficiency is augmented via L-arginine deprivation in HEK-293 cells.," Journal of Zhejiang University. Science. B, vol. 20, no. 12, pp. 1021-1026, December 2019.
[20] F. F. Diehl, T. P. Miettinen, R. Elbashir, C. S. Nabel, A. M. Darnell, B. T. Do, S. R. Manalis, C. A. Lewis and M. G. Vander Heiden, "Nucleotide imbalance decouples cell growth from cell proliferation," Nature Cell Biology, vol. 24, p. 1252–1264, 2022.
[21] G. Ben-Menachem, A. Mousa, T. Brenner, F. Pinto, U. Zähringer and S. Rottem, "Choline deficiency induced by Mycoplasma fermentans enhances apoptosis of rat astrocytes.," FEMS microbiology letters, vol. 201, no. 2, pp. 157-62, July 2001.
Read more
Read less


